Growth Hormone Releasing Peptides
By Richard Walker, M.D.
Ed.- Dr. Walker is the founder of the Scientific Aging Research Academy (SARA) and is one of the serious academic scientists who for many years has supported antiaging research and is an expert in hGH replacement therapy. hGH (human growth hormone) has had a lot of interest in it, even since Dr. Daniel Rudman’s research in the 1980s, whereby he administered injectable GH to elderly individuals and after a 2 to 3 month period stated that many of their biological age markers had been reversed by 20-years! This included less fat tissue, more muscle mass, better skin elasticity and hair condition and a feeling of well-being. As you may imagine, such a result caused a furor, and Dr. Ronald Klatz produced a good book on the subject called; ‘grow young with HGH.’
Since Dr. Rudman’s publication, a lot more research has been undertaken and doses have been reduced somewhat. The recent published research of Dr. Greg Fahy has shown patients supplementing with hGH are able to rejuvenate their thymus gland. This has significant implications for improved immunity and is yet another benefit for this multi-factorial hormone when it is used correctly.
Alas, many countries have decided that GH itself has anabolic properties and therefore must be a controlled substance. Since it is a 191-chain of aminoacids it can only be effective if it is injected. Therefore, many people have been looking for alternatives, both from a legal perspective and from a convenience perspective (i.e. no need to inject). The most promising of these at present are the GHRPs (growth hormone releasing peptides). As Dr. Walker has a lot of experience and knowledge from studying the role of GHRPs and their benefits for aging individuals, we asked him if he would kindly detail the technology and clinical facts behind their use. This article is his detailed reply.
The origin of a novel GH secretagogue
Bowers and Momany synthesized the first growth hormone releasing peptide using a novel approach to determine the then unknown sequence of a growth hormone releasing hormone (GHRH) 1. They modified the structure of small opioid neuropeptides that possessed very weak GH-releasing activity, assuming that structural alteration might produce new peptides with greater efficacy. In fact, GHRP-6 was the first synthetic peptide of the series that elicited dose-related GH release in-vitro and in-vivo2.
Additional modifications were produced to improve the original discovery, of which four emerged as most logical candidates for drug development, including GHRP-6 (His-D-Trp-Ala-Trp-D-Phe-Lys-NH2), Hexarelin (His-D-Mrp-Ala-Trp-D-Phe- Lys-NH2), GHRP-2 (D-Ala-D-β-Nal-Ala-Trp-D-Phe-Lys) or pralmorelin which was most potent, and ipamorelin (Aib-His- D-2-Nal-D-Phe-Lys-NH2) which persisted in the circulation longer than the other GHRPs3 .
Initially, observations of GHRP’s effects were confusing and resulted in their being differentiated from GHRH as a special group called growth hormone secretagogues (GHS) since GH was the first hormone whose secretion was known to be stimulated by them. Although GHS released GH, they did not behave as expected had the new molecules truly been GHRH or analogs of it such as sermorelin. Unlike GHRH, GHS was only weakly effective in vitro when incubated with pituitary tissue. To be effective, it required hypothalamic tissue to be present in the incubate. Paradoxically, when administered to healthy lab animals and later to humans it was more active than exogenous GHRH. This potency was lost when GHS was given to lab animals with hypophyseal stalk section or to human subjects suffering hypothalamic pituitary disconnection as the result of disease or trauma4. This led to speculation that GHS was releasing a heretofore unknown factor (‘U’ factor) from the brain5.
The idea was reinforced by the observation that when GHRP was administered concomitantly with GHRH, its effect to elevate circulating GH was not simply additive to the anticipated responses of GHRH or that of its truncated analog, sermorelin. Instead it potentiated them, suggesting that the putative ‘U’ factor complemented the GH-releasing effects of GHRH and GHS. Lesser potentiation was observed in vitro when GHS/GHRH were coincubated further suggesting that another factor(s) contributed to the potentiation of GHRH by GHS in-vivo. However, it is now known that full potentiation of GHRH in-vivo is due to GHS suppression of somatostatin as well as to stimulation of GHRH releasing neurons in the arcuate nucleus6.
Discovery of ghrelin – Functional differences between GHRH/Sermorelin and Ghrelin/GHRP
GHS enhances GH secretion by different mechanisms and acts through different receptors from those for GHRH, somatostatin or opioid peptides. In 1996, Howard et al. cloned the GHS receptor (GHS-R), which was mainly found in the anterior pituitary and in the hypothalamus, and in other areas of the central nervous system7. While GHS downregulated these receptors, it was incapable of downregulating GHRH receptors in the same tissues. These different receptors initiated different metabolic cascades involving separate second messengers and ion fluxes (Figure 1).
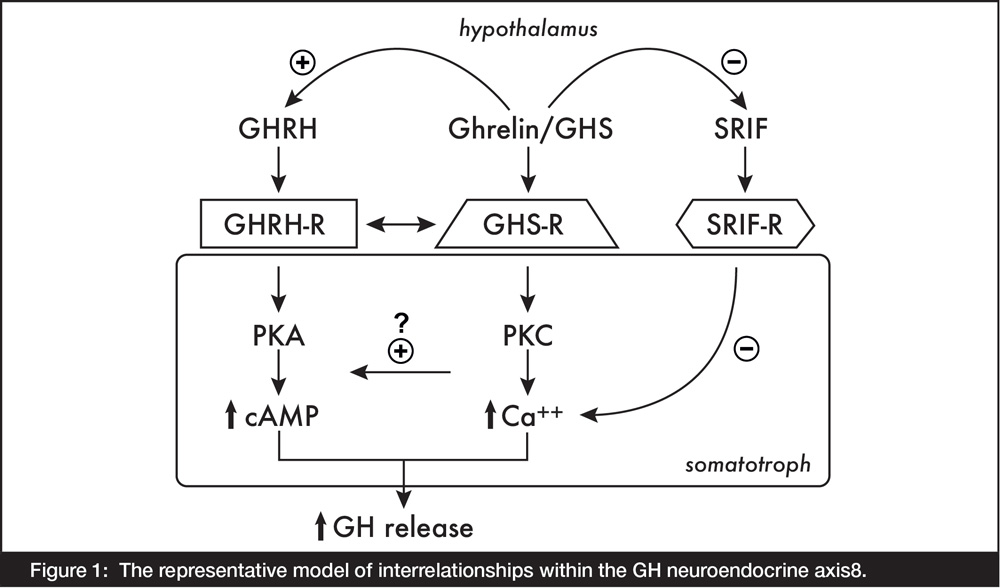
Figure 1: The representative model of interrelationships within the GH neuroendocrine axis8.
The endogenous ligand for these GHS receptors was discovered in the stomach and hypothalamus by Kojima et al (1999), thereby establishing a new brain-gut peptide family. Ghrelin, the name chosen for the new hormone was derived from ghre, the Indo-European root of the word grow 9.
A distinctive quality of GHS/ghrelin differentiating it from GHRH/sermorelin is that depending upon the dose administered, it releases pituitary peptides other than GH, primarily ACTH/adrenocorticoids and prolactin. Through these additional endocrine affects, the synthetic GHS peptides also markedly increase appetite and weight gain, modulate cell proliferation and survival, are cardioprotective, affect heart performance and vascular resistance, are immuno-supportive and anti-arthritic, influence gastric acid secretion and motility, energy/glucose metabolism, as well as exocrine and endocrine pancreatic function.
These properties increase the potential of GHS for extra-GH management of diverse, maladaptive, age-related changes ranging from accumulation of reactive oxygen species (ROS) to cardioprotection (Figure 2).
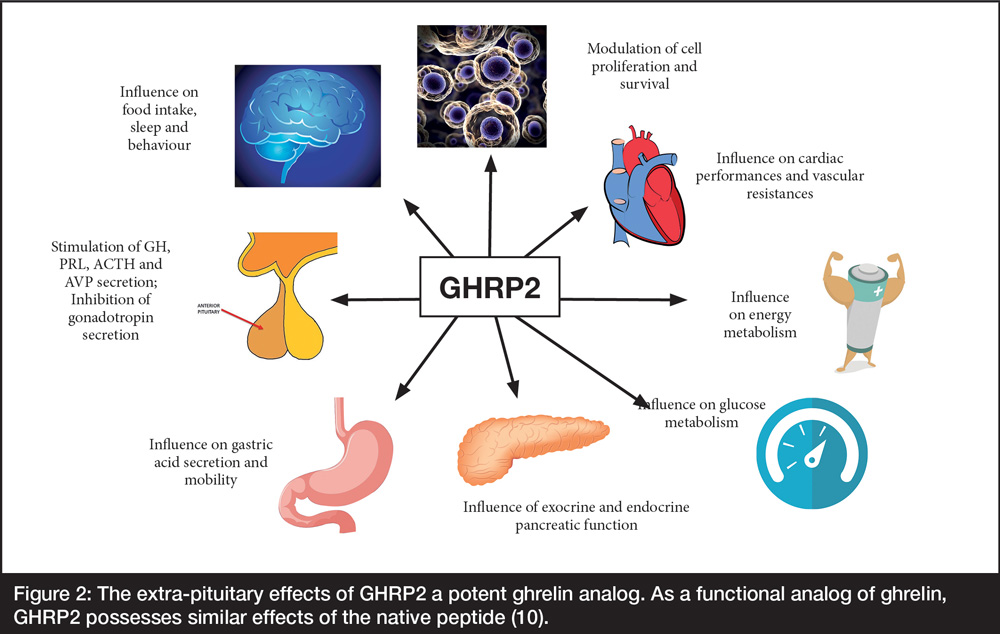
Figure 2: The extra-pituitary effects of GHRP2 a potent ghrelin analog. As a functional analog of ghrelin, GHRP2 possesses similar effects of the native peptide (10).
Structural characteristics favoring oral bioavailability
Characteristic of the entire series of GHRPs, and necessary for their efficacy is the presence of alanine (Ala), tryptophan (Trp) and d-phenylalanine (D-Phe) within their central structure except for ipamorelin, the last in the series which lacks Ala and Trp, but retains D-Phe (Table 1).
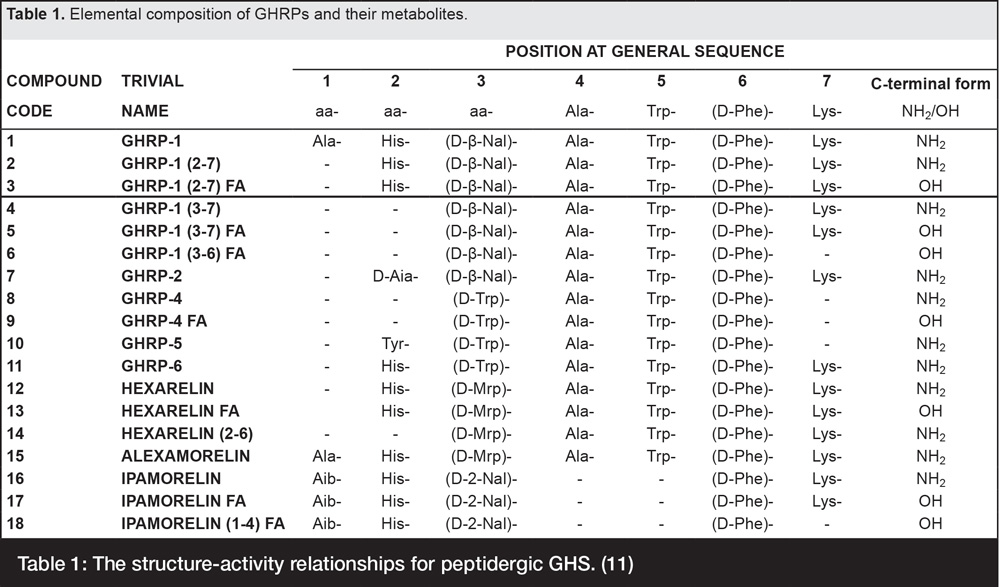
Table 1: The structure-activity relationships for peptidergic GHS. (11)
The presence of d-amino acids blocking each side of the internal, activity essential, tri-peptide moiety is a functionally significant attribute of all GHS peptides because they protect the molecules from endopeptidase attack and thus, make them orally bioavailable12. As shown in Figure 3, endopeptidases cleave peptides internally and thus, would have the potential to destroy the active moiety in GHS were it not protected by the d-amino acid isomers.
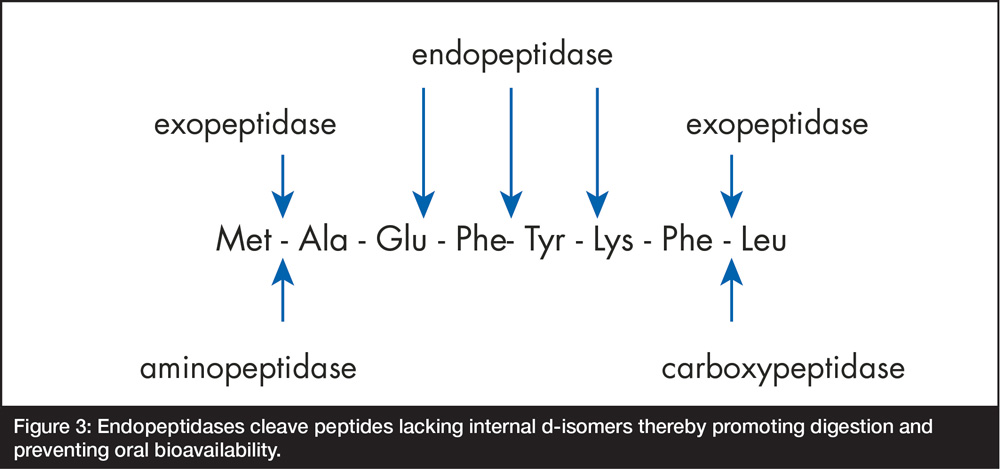
Figure 3: Endopeptidases cleave peptides lacking internal d-isomers thereby promoting digestion and preventing oral bioavailability.
As a result of their oral bioavailability, GHRP2 and other GHS are now available as sublingual tablets that are a more convenient dosage form for daily use at home and especially during travel. A popular commercial brand of this dosage form is GHRP2Pro™ that is now available from IAS online.
Drug Development
Despite the multiple benefits of GHS and the fact that they are safe, (no serious general pharmaco-pathological effects at dose levels showing GH-releasing activity have ever been reported), extensive basic research by university and other public sector scientists undoubtedly thwarted commercial development due to loss of patent protection and other competitive factors.
However, some drug development was attempted, albeit unsuccessfully. For example, commercial development of GHRP-6 as a feed additive to improve milk production in cows was attempted by SmithKline Beecham during the mid-to late 1980’s but was never approved for use. Since GHS is orally bioavailable, the logic for this application was that it could be added in relatively large amounts to feed for dairy cattle. Directly relevant to the marketing attempt was the secondary effect of the peptides to stimulate prolactin release and thereby, potentially increase milk production.
GHRP-2 (pralmorelin) was also under investigation for the treatment of growth hormone deficiency (GHD) and short stature (pituitary dwarfism), and was advanced to phase II clinical trials for these indications, but ultimately, was never marketed for them13.
There have been few other specific drug development efforts to exploit clinical applications for GHS except as a diagnostic. Kaken-Japan, developed and received approval of GHRP-2 as a minor market product, i.e., a single dose formulation for assessment of adult GH deficiency14. This diagnostic application is the only currently regulated drug indication being marketed.
Quantitative benefits of GHRP2 in aging
Lacking extensive development of GHRP2 as a ‘big Pharma’ drug product, it became available for ‘off label’ application in anti-aging medicine. Logically, the medical basis for such applications derived from the positive effects of recombinant human growth hormone (rhGH) on phenotype, e.g., increasing lean body mass, decreasing abdominal adiposity, improving skin tone, etc. as first reported by Rudman15.
However, legal restrictions preventing its use for anti-aging purposes led to a search for other effective and safe means of GH replacement therapy (GHRT).
Any GH stimulating component of the GH neuroendocrine axis can be used for GHRT with some degree of benefit. However, these are also potentially detrimental even though they bring some level of somatic rejuvenation. Unfortunately, hormones that act directly within the GH neuroendocrine axis can also degrade functional/physiological interactive relationships thereby exacerbating age-like, maladaptive changes. For example, somatomedin (IGF-1 et al.) exerts negative and positive, long-loop feedback on GHRH and SRIF, respectively. Similarly, though negative, and positive feedback, rhGH essentially shuts down its own endogenous synthesis while stimulating SRIF release, depending upon the dose administered.
To attempt to circumvent this problem, sermorelin, a truncated analog of GHRH has been used to avoid negative feedback effects of rhGH and/or IGF-1 that can diminish production of intrinsic hGH by the adenohypophysis. However, ultra-short negative feedback involves GHRH/sermorelin upon its own endogenous production and positive feedback on SRIF producing neurons. Thus, these approaches limit their efficacy which wanes after extended use, indicating that use of specific hormones within the GH neuroendocrine axis for somatic benefits of GHRT should be weighed against their dysfunctional, physiological/neuroendocrine potential.
In contrast to the specific effects of GHRT by peptides directly within the GH neuroendocrine axis, GHS enhances secretory performance because it mitigates the progressive, maladaptive dominance of intrinsic factors such as sermorelin/GHRH and somatostatin (Figure 4).
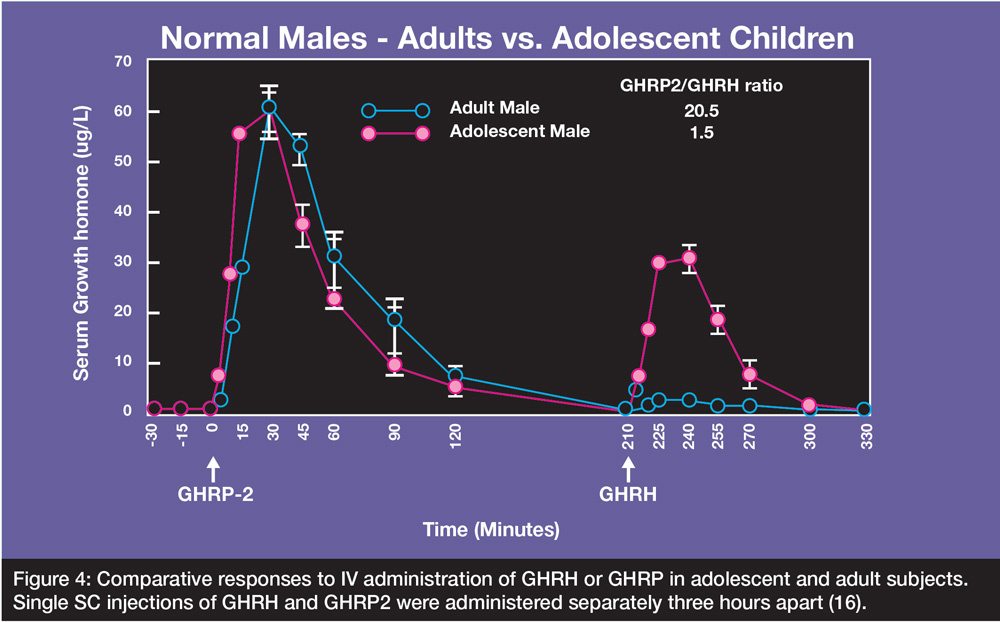
Figure 4: Comparative responses to IV administration of GHRH or GHRP in adolescent and adult subjects. Single SC injections of GHRH and GHRP2 were administered separately three hours apart (16).
As seen in Figure 4, GH release in response to GHRP2 was comparable in adolescents and middle-aged, adult subjects. In contrast, the response to GHRH was only modestly reduced in adolescents when compared with that of GHRP2. However, it was dramatically attenuated in the adult subjects. Thus, the data suggest that the synergistic response to GHRP2 in-vivo, is not dose related to endogenous GHRH, but rather just requires the presence of some quantum of the natural hormone to realize a significant part of its full stimulatory potential.
The response to GHRP2 in adults also suggests that rapid recrudescence of GHRH neuronal elements may be evoked by the ghrelin analog. In contrast, the minimally additive effect of exogenous GHRH with endogenous GHRH in the adult subjects reflects an age-related decline in the latter neuropeptide and of increased somatostatin tone with advancing age17.
In the long term, by stimulating GHRH neurons, GHS sustains its receptors by direct effect and also due to association with dopamine receptors to form GHSR1a:DRD2 heteromers18. It also inhibits SRIF neurons, prevents desensitization of the GHRH receptor (GHRH-R)19-21 and reduction of GHRH-R messenger RNA accumulation22. Pituitary recrudescence is also facilitated by GHS because it recruits separate somatotrophs from its three subpopulations23. Through these effects, GHRP2 enhances efficacy of endogenous GHRH through synergy and promotes ‘feed forward’ that overrides negative feedback factors upon GHRH neurons. Since GHS enhances ‘feed forward’ by activating GHRH neurons essentially from outside the main axis, it shifts activity relationships thereby tending to support expression of GH secretory pulses which are a dominant characteristic of youthful animals and humans.
Qualitative benefits of GHRP2 in aging
Neuroendocrine aging represents a gradual process during which patterns of GH secretion that are modulated in young, healthy people, degrade slowly over time. During youth, normal GH secretion is expressed as minimal, basal serum concentrations upon which occur highly regulated pulses of the hormone. These pulsatile as well as nychthemeral secretory patterns are pre-eminent physiological features of healthy GH release and are critical to physiological regulation and long-term activation of the GH neuroendocrine axis. Such pulsatile signaling resulting from intermittent release of GHRH from neurosecretory hypothalamic neurons consistent with prior and concurrent hypothalamic somatostatin tone preserves biosynthesis and secretion of somatotropin. Pulsatile GH signals also activate organ-specific second messenger signaling pathways, such as the Signal Transducer and Activator of Transcription 5B (stat5B) in the liver, with subsequent induction of specific genomic responses in relevant target tissues. STAT5b is a protein coding gene that affects sensitivity to GH. These are not equivalently induced by constant or disorganized GH stimuli. Thus, for anti-aging purposes, restoration of frequent, regularly occurring GH pulses is a very important physiological effect because it represents recrudescence of spontaneous GHRH neuronal activity bursts, enhancing the quality of feedback control dynamics.
As previously shown, the potential quantitative benefits of GHRP2 derive from its robust ability to stimulate large amounts of GH from the pituitary. And its physiological/qualitative benefit results from its ability to restore spontaneous GHRH neuronal activity bursts that enhance feedback control dynamics and maintain pulsatile release of hGH from the pituitary gland.
As shown in Figure 5, restoration of GH pulses in older men and women was accomplished clinically using GHRP-2 but not comparable doses of GHRH or its truncated analog, sermorelin. These responses represent restoration of feedback relationships and increased coupling with interdependent neuroendocrine components.
The latter effects are demonstrated by relatively greater increase in IGF-1 after 30-d stimulation with GHRP-2. Presumably, similar effects can be achieved by anti-aging physicians using commercially available GHRP2Pro™.
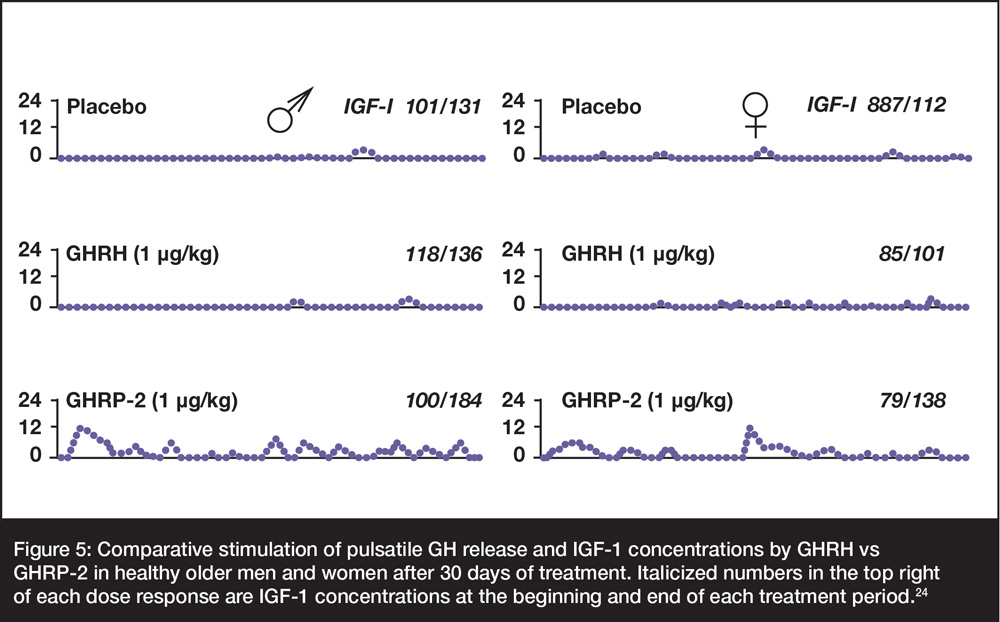
Figure 5: Comparative stimulation of pulsatile GH release and IGF-1 concentrations by GHRH vs GHRP-2 in healthy older men and women after 30 days of treatment. Italicized numbers in the top right of each dose response are IGF-1 concentrations at the beginning and end of each treatment period.24
Age-changes in GH secretory dynamics at later stages of life are clinically measurable as frank alterations of the earlier patterns that can be quantified by measurement of pulse timing and amplitude. However, the subtle slowly varying features of such pulsatile change during the onset and early stages of senescence cannot be easily delineated quantitatively by such measurement. Thus, decay of earlier, organizational aspects of GH release can be quantified using the statistic, approximate entropy (ApEn)25 which quantifies the persistence of (sub)pattern features in repetitive measurements over time 26,27.
Notably, as applied to endocrine time-series, ApEn will detect alterations in underlying episodic behavior that are not reflected in peak occurrences or amplitudes, a point germane to many applications. In fact, ApEn statistical applications have unmasked consistent and significantly greater secretory disorderliness with advancing age for the GH axisproviding a direct barometer for the strength of the feedback system28. Such data would be important for predicting the state of neuroendocrine aging and theoretically as interventions become available, be valuable in knowing when to begin therapy. Since GHRP2 enhances ‘feed forward’ by activating GHRH neurons from outside the main axis, and thereby opposes age-associated degradation of regulatory control within the GH neuroendocrine axis, it is a logical consideration for GHRT by practitioners of anti-aging medicine.
Conclusion
Because of its feed-forward effects and oral bioavailability, GHRP2 hormone replacement may provide a more holistic approach to GH-directed therapy. Compared with others, its more effective, beneficial, and physiological outcomes for hGH neuroendocrine health and vitality can be expected from GHRP protocols such as those utilizing GHRP2Pro™.
By using peptides such as GHRP2, in conjunction with other established anti-aging interventions, then good physical and functional outcomes may be realized.
References
- Bowers CY. (2000) GHRP Historical Perspective Basic and Clinical. In: Human Growth Hormone Basic and Clinical Research. Contemporary Endocrinology, eds R. Smith, M. Conn, Humana Press, New York, page 17-43.
- Bowers CY, Momany FA, Reynolds GA, Hong A. (1984) On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology, 114(5):1537–1545.
- Raun K1, Hansen BS, Johansen NL Et al. (1998) Ipamorelin, the first selective growth hormone secretagogue. Eur J Endocrinol, 139(5):552-561.
- Pandya N, DeMott-Friberg R, Bowers CY et al. (1998) Growth Hormone (GH)-Releasing Peptide-6 Requires Endogenous Hypothalamic GH-Releasing Hormone for Maximal GH Stimulation, J Clin Endo Metab, 83(4): 1186– 1189
- Bowers, CY. (1998) Growth Hormone Releasing Peptide (GHRP). Cell and Mol Life Sci. 54(12):1316-1329.
- Cella SG, Locatelli V, Poratelli M, et al. (1995) Hexarelin, a potent GHRP analogue: Interactions with GHRH and clonidine in young and aged dogs. Peptides, 16 (1):81-86.
- Howard AD, Feighner SD, Cully DF, et al. (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996, 273:947-977.
- Lengyel AMJ (2006) From growth hormone-releasing peptides to ghrelin: discovery of new modulators of GH secretion. Arq Bras Endocrinol Metab [online]. 2006, vol.50, n.1 [cited 2019-11-11], pp.17-24.
- Kojima M, Hosoda H, Date Y, et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature, 402:656-60.
- van der Lely AJ et al. (2004) Biological, Physiological, Pathophysiological, and Pharmacological Aspects of Ghrelin. Endocrine Reviews 25(3):426–457.
- Ferro P. et al. (2016) Structure-activity relationship for peptídic growth hormone secretagogues. Drug Test. Analysis, 9(1):1-5.
- Walker et al. (1990) Oral activity of the growth hormone releasing peptide His-D-Trp-Ala-Trp-D-Phe-Lys-NH2 in rats, dogs and monkeys. Life Sciences, 47:29-36.
- Adis Editorial (2004). “Pralmorelin”. Drugs in R&D. 5 (4): 236–239. doi:10.2165/00126839-200405040-00011
- Graul, A I, Prous, JR (2006). “The Year’s New Drugs: A Historical and Research Perspective on the 41 New Products that Reached their First Markets in 2005”. Drug News & Perspectives. 19 (1): 33.
- Rudman D, Feller AG, Nagraj HS et al. (1990) Effects of human growth hormone in men over 60 years old. N Engl J Med. 323(1):1-6.
- Walker RF, Bercu BB (1998) Comparative effects of GHRH and GHRP2. JAAM 1:219-225, 1998
- Spik K, Sonntag WE, (1989) Increased pituitary response to somatostatin in aging male rats: relationship to somatostatin receptor number and affinity. Neuroendocrinology 50:489-494
- Kern A, Grande C, Smith RG. (2014) Apo-ghrelin receptor (apo-GHSR1a) regulates dopamine signaling in the brain. Front Endocrinol 5: 1-8. doi: 10.3389/fendo.2014.00129
- Hansen BS et al. (2001) The Growth Hormone-Releasing Hormone Receptor: Desensitization Following Short-Term Agonist Exposure. Pharmacology & Toxicology, 88, 81–88.
- Bilezikjian, L.-M., Seifert H, Vale W: (1986) Desensitization to growth hormone-releasing factor (GRF) is associated with downregulation of GRF-binding sites. Endocrinology 118:2045–2052.
- Vance, M. et al. (1986) Dual effects of growth hormone (GH)-releasing hormone infusion in normal men: somatotroph desensitization and increase in releasable GH. J. Clin. Endocrinol. Metab. 62: 591–594.
- Aleppo G, et al. (1997) Homologous down-regulation of growth hormone-releasing hormone receptor messenger ribonucleic acid levels. Endocrinology.138(3):1058-1065.
- Mitani M, Kaji H, et al. (1996) Growth hormone releasing peptide and GH releasing hormone stimulate GH release from subpopulations of somatotrophs. J Neuroendocrinol. 8(11):825-830.
- Bowers CY, Granda R, Mohan S et al., (2004) Sustained Elevation of Pulsatile Growth Hormone (GH) Secretion and Insulin-Like Growth Factor I (IGF-I), IGF-Binding Protein-3 (IGFBP-3), and IGFBP-5 Concentrations during 30-Day Continuous Subcutaneous Infusion of GH-Releasing Peptide-2 in Older Men and Women. J Clin Endocrinol Metab 89:2290–2300
- Pincus, S. M.; Gladstone, I. M.; Ehrenkranz, R. A. (1991). “A REGULARITY STATISTIC FOR MEDICAL DATA ANALYSIS”. Journal of Clinical Monitoring and Computing. 7 (4): 335–345. doi:10.1007/BF01619355
- Pincus SM. Approximate entropy as a measure of system complexity (statistic/stochastic processes/chaos/dimension). Proceedings of the National Academy of Sciences of the USA 1991 88 2297–2301.
- Pincus SM & Kalman RE. (1997) Not all (possibly) ‘random’ sequences are created equal. Proc Nat Acad Sci. 94:3513–3518.
- Veldhuis JD, Pincus SM, (1998) Orderliness of hormone release patterns: a complementary measure to conventional pulsatile and circadian analyses Eur J Endo, 138:358–362
