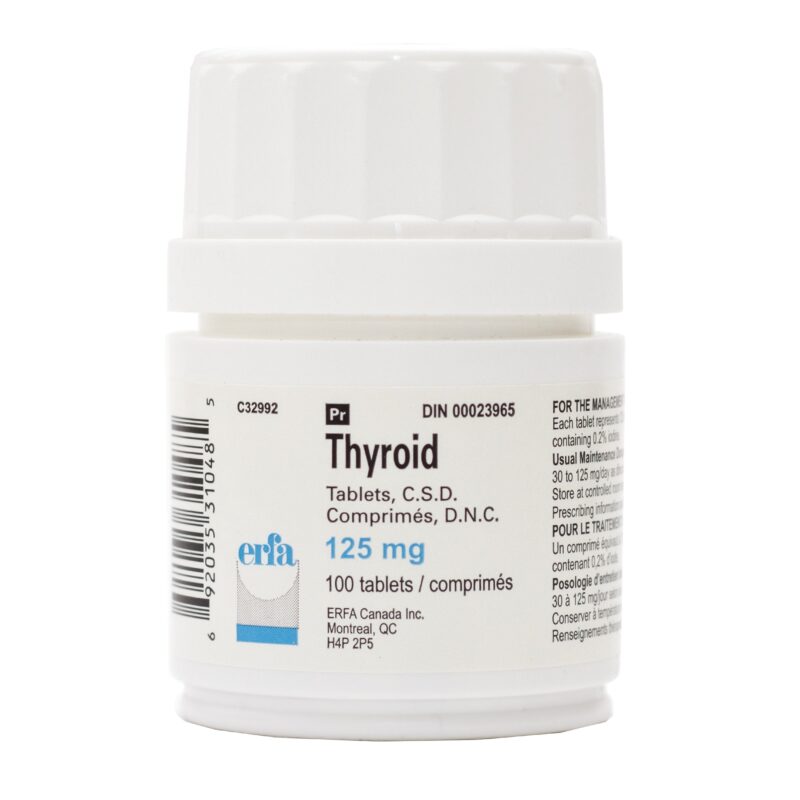
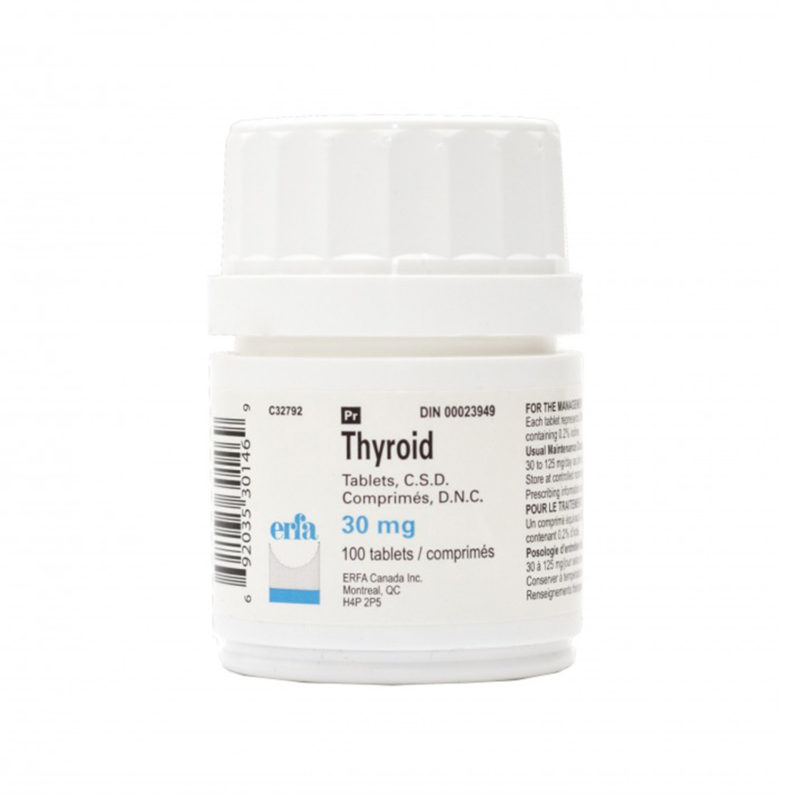
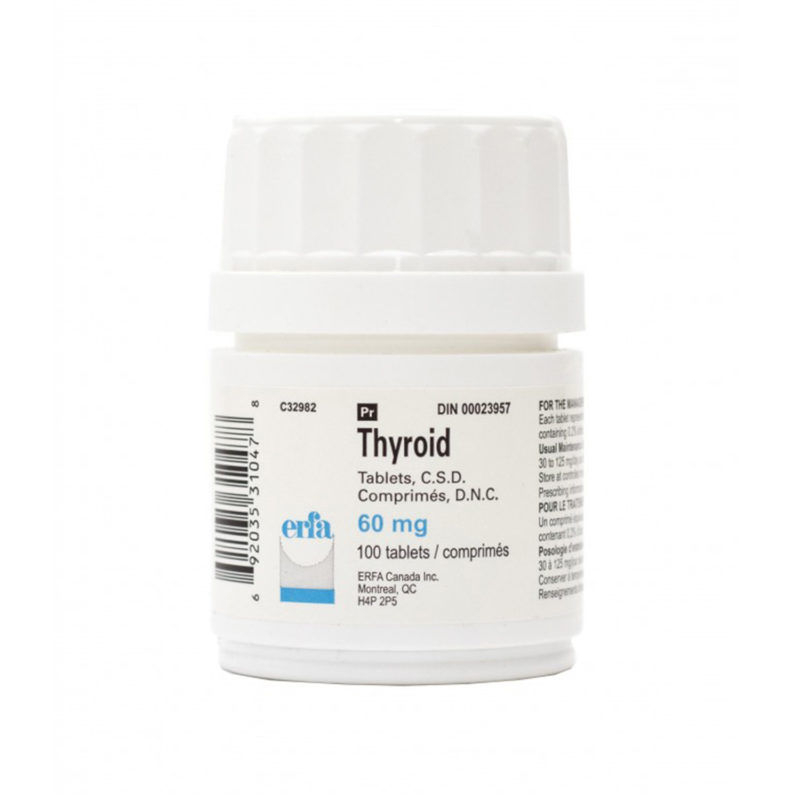
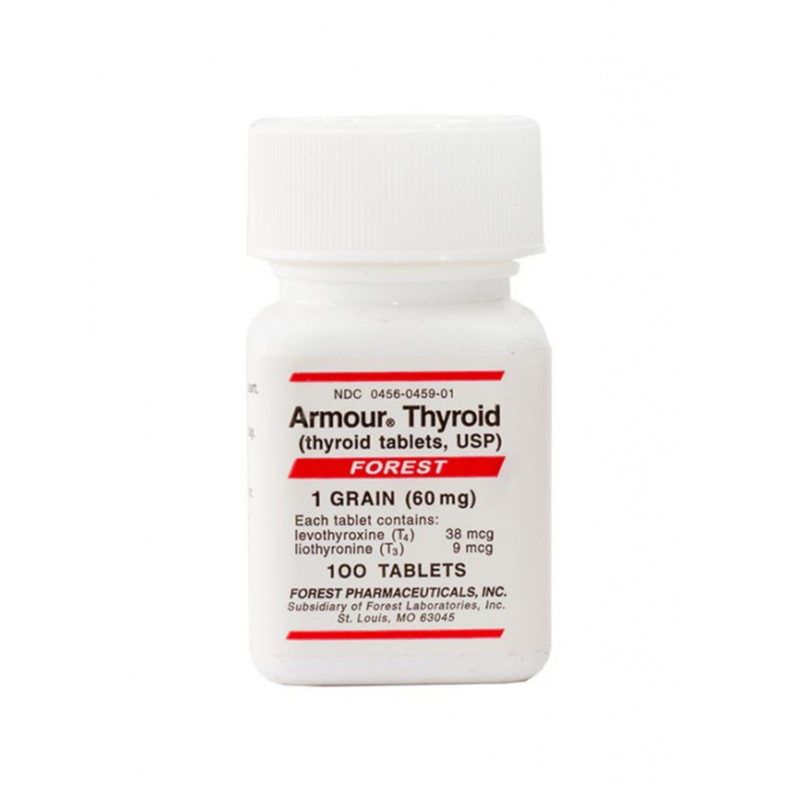
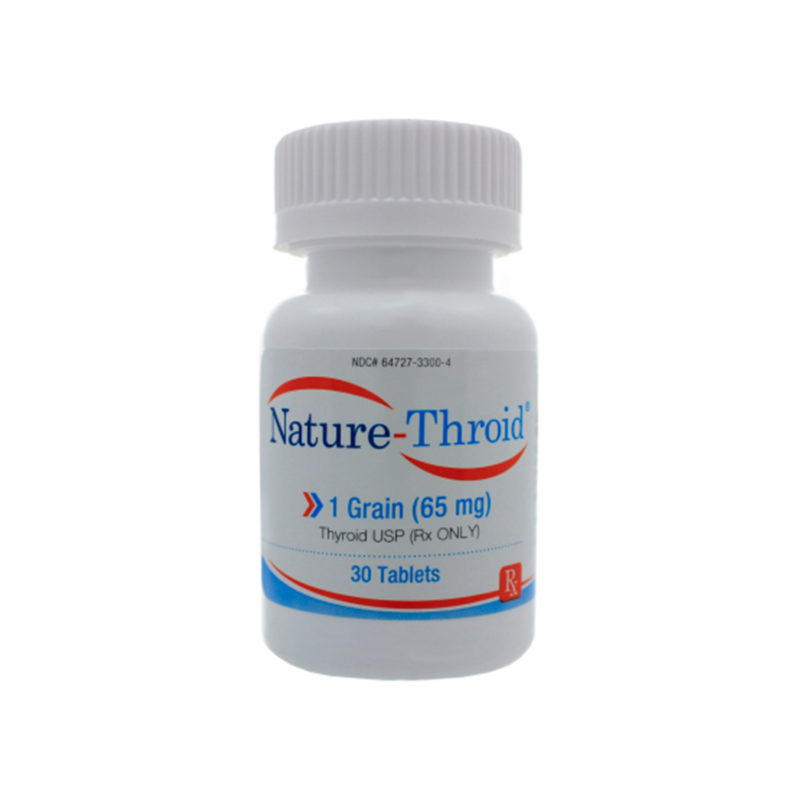
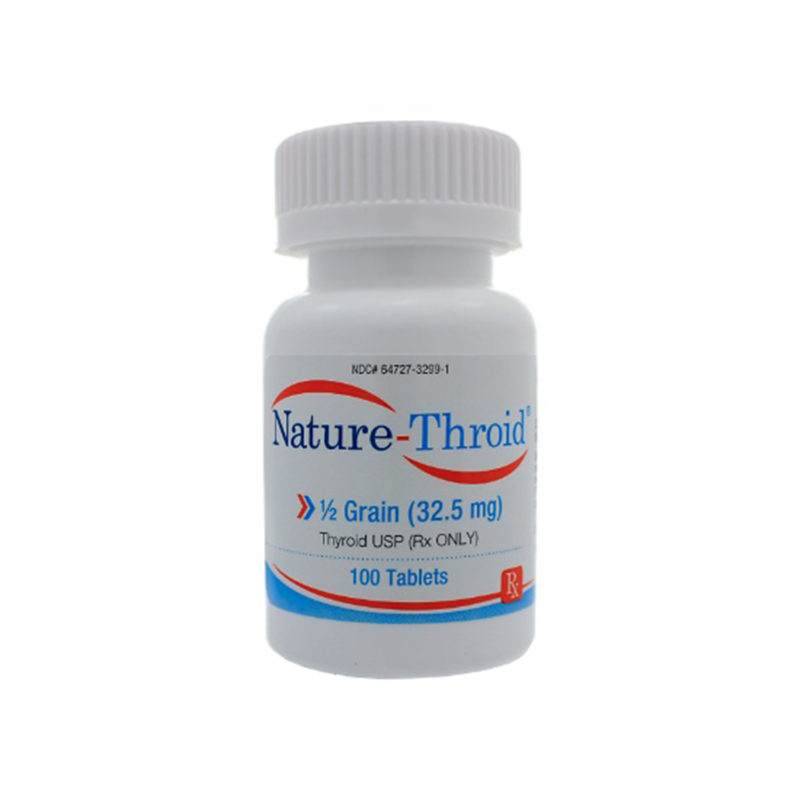
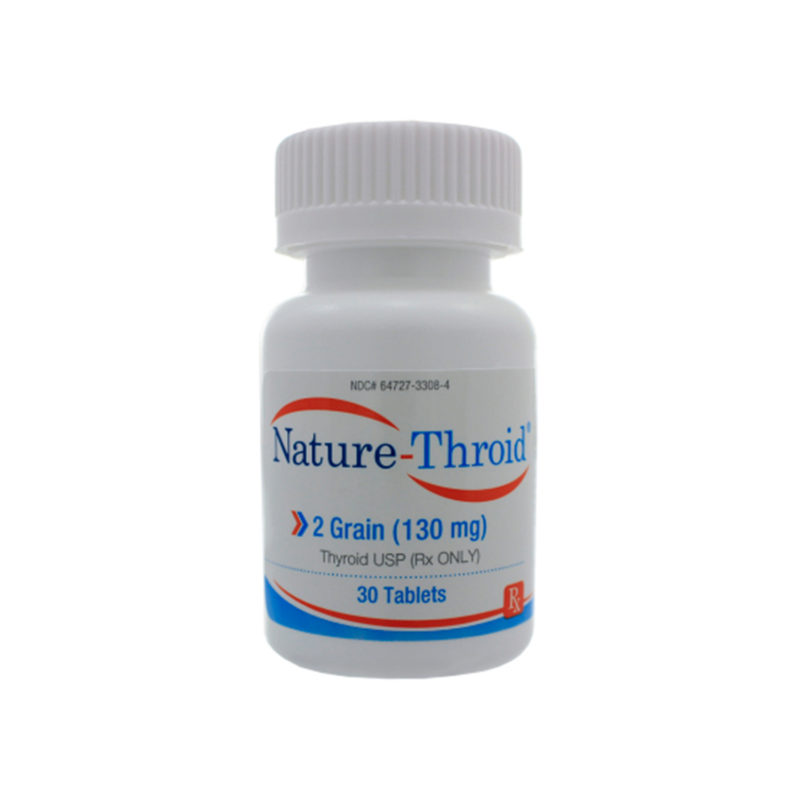
Desiccated thyroid
Desiccated thyroid extract is a dried natural substitute for conventional, synthetic thyroid medications. It is made from dried animal thyroid glands, most often porcine, and is most often used in the treatment of conditions that are caused by low thyroid hormone – this is where the thyrpid gland does not produce enough thyroid hormone (TSH).
Desiccated thyroid extract medications do not only contain T4, they also contain the other thyroid hormones T2 and T3. There are a number of different versions including Nature® , ERFA® and Armour®.