Brace Yourself! Advances in Rejuvenation Research Are Just Around the Corner
During the early 1860s, the popular French author Jules Verne wrote a novel about the distant future of Paris. In his 1863 book, Paris in the Twentieth Century (1), Verne predicted the coming of skyscrapers, the subway, a primitive version of the Internet, automobiles and a manned trip to the moon.
How did he achieve such remarkable foresight? He accurately predicted those scientific and engineering achievements by meeting and talking with leading scientists of his time.
I believe it is possible to predict advances thirty years hence by talking with leading thinkers of our time. For example, during the next few decades, insurance company consultants have estimated that auto insurance policyholders will drop by at least 40 percent because most cars will be self-driven, leading to fewer accidents and eliminating human errors (2). “We think that over the next 20 to 25 years, the number of accidents will fall by 80 percent,” said Jerry Albright, principal actuarial of insurance risk practice at KPMG, the consulting firm that released the report.
Antiaging Medicine Fully Implemented in the Future
After interviewing several prominent scientists, I predict that a similar percentage drop in health insurance will occur in coming decades when antiaging medicine is fully implemented and enforced by HMOs and Medicare. These agencies are currently faced with ever-increasing medical reimbursements and health-care premiums that should decrease dramatically when most diseases are prevented or delayed. Practically, implementing antiaging medicine means that we will be preventing cancer, heart disease, and other major diseases instead of waiting for them to strike and, consequently, keeping health insurance companies or the government from paying millions of dollars for expensive drugs.
To address this future goal of antiaging prevention, the American Academy of Anti-Aging Medicine (A4M) aims to address this future goal of anti-aging prevention. A4M is a nonprofit medical society, dedicated to the advancement of technology to detect, prevent, and treat age-related disease and to the research of methods to retard and optimize the human aging process (3). Since the founding of A4M in 1992 by Ward Dean, MD, and others, significant strides have been made toward a slowed-aging or no-aging future—some might even say a reverse-aging future—with major diseases being cured or at least managed. In my opinion, this future will likely occur in the next couple of decades.
One such example of recent antiaging advances involves ameliorating or even reversing aging-related hearing loss.
Breakthroughs in the Science of Hearing Loss Caused by Aging
“In the USA, 63% of Americans older than 70 have hearing loss” (4)
Hearing loss is a common disability that is often attributed to aging. It is also caused by drugs, loud noise, and genetics. In the United States, 63% of Americans older than 70, have some degree of hearing loss. (4) Severe early-onset deafness is found in 4–11 per 10,000 children, and genetic defects cause 50 percent of all cases (5).
Despite the high incidence of hearing loss, no effective treatment has been established except for cochlear implants or steroid treatment such as the hormone aldosterone. Most hearing loss cases are caused by the loss or functional impairment of hair cells (Figure 1) in the inner ear’s cochlea, but scientists have not believed that hair cells proliferate after an infant’s birth (6). This fact has prevented the development of effective treatment methods for human hearing loss despite evidence that hair cells can regenerate in birds.
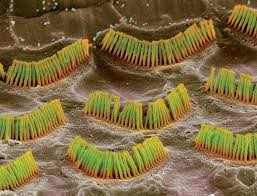
Figure 1: Hair cells in the inner ear’s cochlea become defective causing hearing loss.
Aldosterone Critical to Hearing
Aldosterone is a hormone that is vital to the aging process. Thus, low aldosterone is often considered to be a biomarker of aging. Symptoms of depletion of this important biomarker are crows’ feet, dizziness, low blood pressure, thirst, dehydration, and salt cravings (7). Many of these symptoms result in an imbalance of the potassium–sodium ratio that leads to fatigue and cellular energy loss in the inner ear’s approximate 15,000 hair cells that are located in the cochlea.
Externally, signs of low aldosterone can be seen in dehydrated people through fine lines, pinched faces, sinuous necks, and a profound daily need for cosmetic lotions that moisten their skin superficially (7). Thus, a multibillion-dollar cosmetic industry continues selling its moisturizing and antiwrinkle creams while the underlying medical problems—namely, low levels of aldosterone and other hormones—remain untreated.
How has this research developed? Apparently, Harvard graduate Jonathan W. Wright, MD, has worked with hearing-deficient patients since 2005 when he first noticed a hearing improvement in a patient named “Tom” (8). Since then, Dr. Wright has been frustrated by the fact that his natural remedy—consuming aldosterone capsules orally—does not always cross over from the bloodstream to the cochlea. Aldosterone must cross over to maintain a 1000:1 gradient of cochlea versus bloodstream aldosterone. Without this extreme gradient, hearing is impaired (8).
Indeed, this problem is similar to the difficulties encountered by many conventional medications in crossing over from the bloodstream to the brain.
A New Method Using Aldosterone Spray
During recent years, I developed a new method to penetrate the inner ear and cochlea directly with aldosterone and achieve that 1,000:1 gradient (Figure 2). When this topical solution is applied and absorption occurs, aldosterone goes to work by better controlling sodium and potassium levels in those delicate sound-detecting hair cells.
Aldosterone carefully balances sodium and potassium without synthetic drugs because an exact balance of these two electrolytes allows the neural transmission of sound signals directly to the brain. This balanced neural transmission of sound signals is typically measured at approximately 50 to 80 millivolts (mV) in strength (9–13).
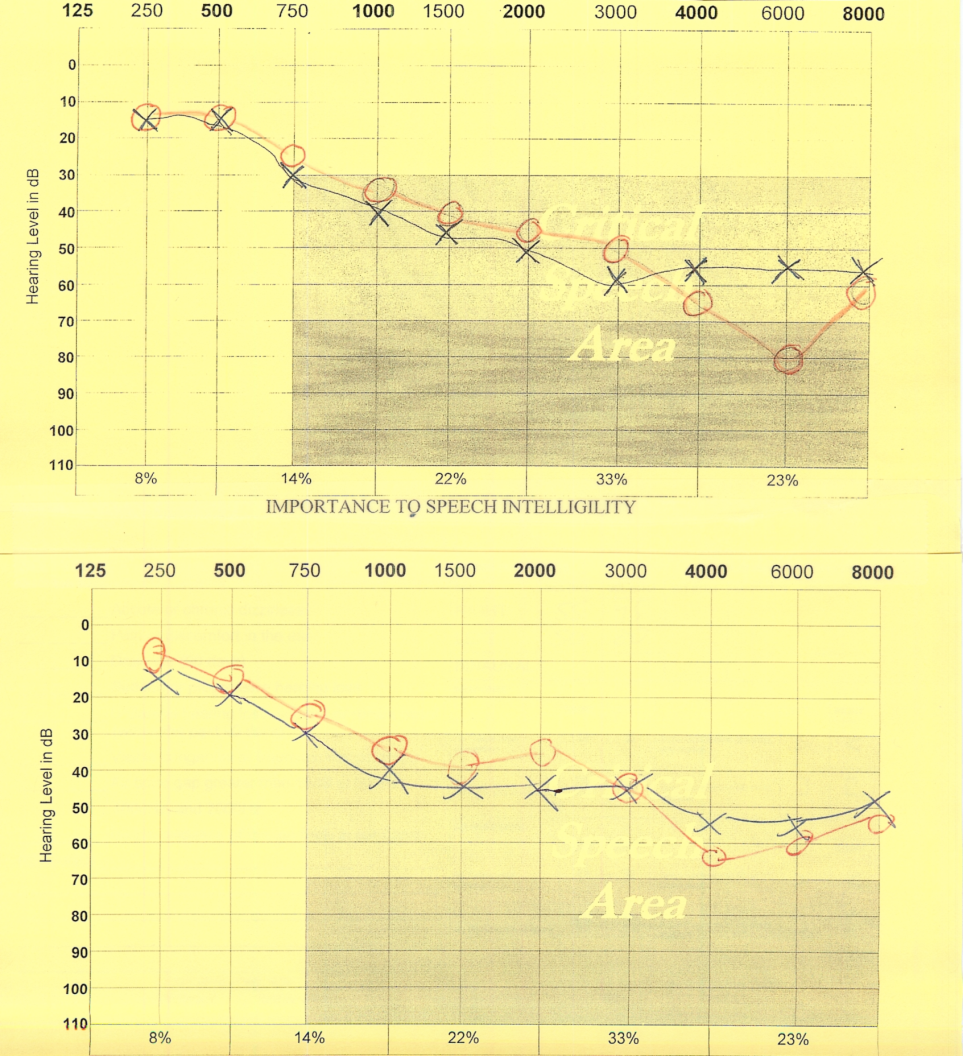
Figure 2: The top audio-graph lines show age-related hearing loss. The bottom lines highlight when volunteers sprayed 125 mcg of aldosterone into each ear canal, their hearing improved by approximately 33% after two hours.
Conversely, patients with deficient aldosterone levels and hearing often register below 50 mV. The higher level of 80 mV allows the brain to interpret nuanced sounds successfully, arriving at the rows of long and short hairs in the ears’ cochlea.
Thus, by correcting for aldosterone deficiencies, hearing improves by up to a resounding 30 decibels, but word recognition is only marginally improved.
As Dr. Wright said, “I’ve witnessed an 87-year-old man who’d lost his hearing 13 years prior to regaining a significant degree of it using aldosterone”(8).
AldoSpray™: A Time-Release Aldosterone Spray for Improved Sound Volume
AldoSpray™is easy to use. Upon waking, simply tilt your head to each side and spray a single dose of aldosterone into each ear canal. Wipe off excess fluid with a paper towel. Most people will notice some hearing improvement after about one hour; hearing improvement of approximately 30–40 decibels occurs two to three hours after application. For many people, hearing continues to improve throughout the day. In addition, tinnitus subsides throughout the day, but it returns at night when aldosterone rapidly metabolizes and the body returns to a deficient level. Before bedtime, you should gently clean your ear canals by using a cotton swab or by washing them out with soap and water.
A Second Hearing Loss Remedy
As stated previously, most hearing loss cases are caused by the degradation or functional impairment of hair cells in the cochlea. Unfortunately, scientists do not believe that human and mammalian hair cells are capable of proliferation after an infant’s birth (6).
However, since the emergence of regenerative medicine in the twenty-first century, new methods have evolved to revive and rejuvenate the inner ear’s human hair cells. One new method uses tissue-engineering technology, one of the most powerful tools of regenerative medicine. This method involves isolating cells and inducing them to grow on a scaffold or matrices with the help of essential growth factors (14). Such growth factors have demonstrated their ability to regulate, proliferate, and differentiate various types of cells in various organs (15, 16). These growth factors include erythropoietin and granulocyte-colony stimulating factors, which have been clinically used to treat anemia and neutropenia.
Other factors such as epidermal growth factor, transforming growth factor alpha, basic fibroblast growth factor, and IGF-1 (insulin-like growth factor) were shown to grow hair cells in neonatal mice cochlear cell culture (17). In particular, IGF-1 acts to induce the anabolic and mitogenic effects of growth hormone (18). IGF-1 exerts its action through its receptor IGF1R, which, in turn, induces phosphorylation on the amino acid tyrosine (19), leading to the formation of membrane-associated phosphorylated inositols. This effect is an important pathway to the proliferation of cells (20), the avoidance of cell death (21), and the beneficial transcription of DNA (22, 40).
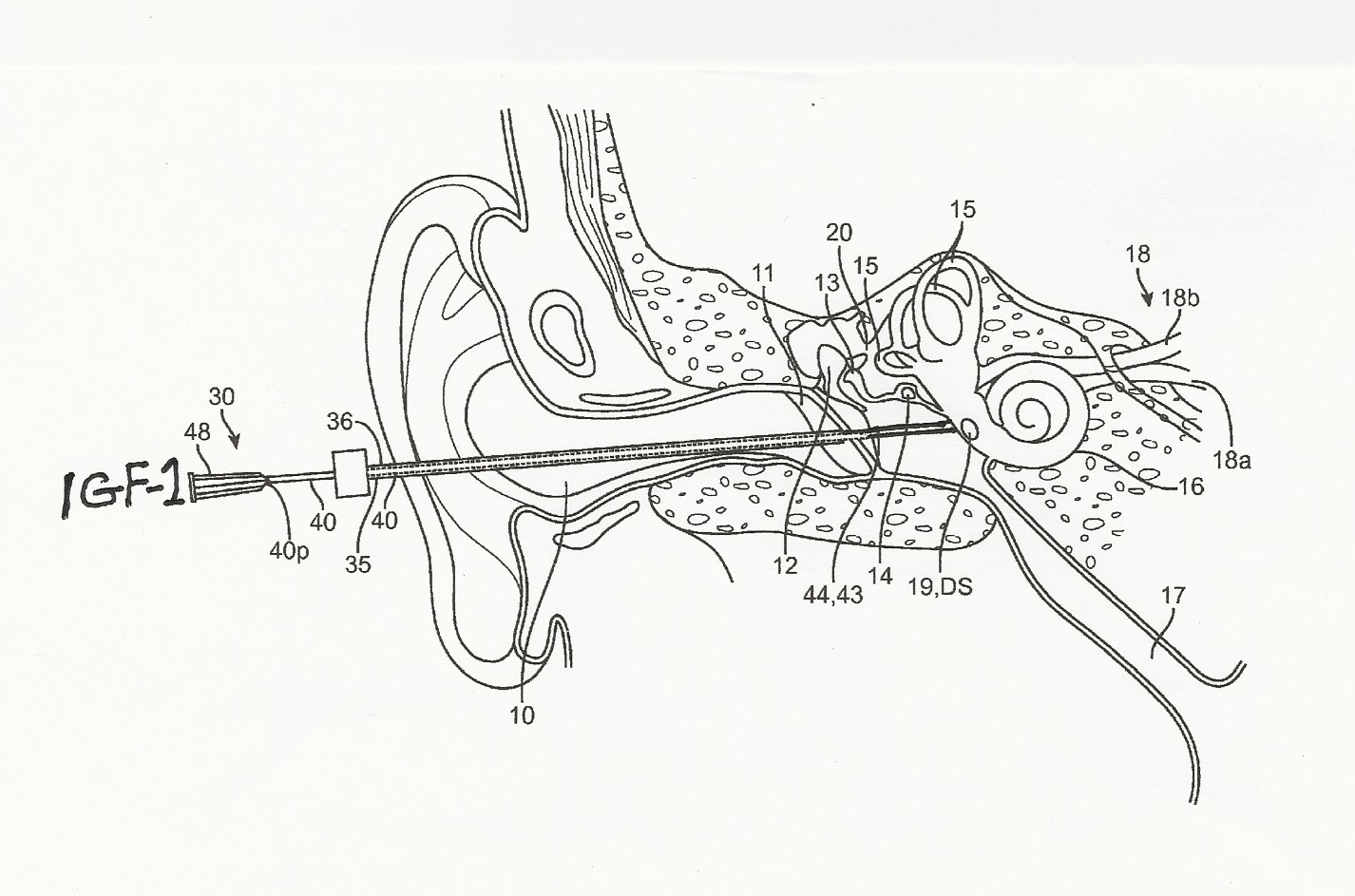
Figure 2: IGF-1 and stem cells dissolved in hydrogel were injected directly into the cochlea of hearing-impaired patients. Result: Even totally deaf patients gained an average of ten decibels of hearing (41).
IGF-1 also causes the anabolic growth of various organs; regulates neuronal survival, neurogenesis, angiogenesis, and excitatory and inhibitory neurotransmission in the central nervous system; and regulates food intake and cognition (23).
Hearing loss occurs in patients with mutations in the Igf1 gene (24, 25, 26) or who have a deficiency of IGF-1 (27) or low IGF-1 serum levels (28, 29).
Indeed, IGF-1 deficiency is known to cause malformation of the inner ear organs and reduced proliferation of inner ear cells such as otocysts (30). Thus, IGF-1 is extremely important to both human and mammalian hearing development and maintenance. It is also essential for its anti-apoptotic effects, and thus, its physiological signaling causes the regeneration of dying or poorly functioning hair cells.
In vivoexperiments with a mature guinea pig inner ear infused with a mixture of IGF-1 and retinoic acid after chemical injury demonstrated complete recovery of hair cells (31).
The half-life of IGF-1 is only eight to sixteen hours (32). Very little of IGF-1 is absorbed into the inner ear from the bloodstream, and the amount is only one ten-thousandth to one hundred-thousandth that of IGF-1 in the bloodstream. Thus, a more effective treatment uses topical carriers such as hydrogel (33). Dr. Iwai and colleagues (34) demonstrated in the mature rat that outer hair cell loss was significantly milder in the cochleae in the IGF-1-treated group versus controls.
Other groups of scientists (Fujiwara et al., 2008; Hakuba et al., 1997; Koga et al., 2003) showed that IGF-1 can positively affect both early- and late-phase hearing damage, and it also has the ability to protect both inner and outer hair cells from damage caused by noise or ischemia. Also, Hayashi et al. (2013; 37) demonstrated that IGF-1 induces cell proliferation in mammalian hair cells and in adjacent cells such as Hensen’s and Claudius’s cells.
In clinical trials, Nakagawa and his group (38, 39) tested twenty-five patients with hearing loss. They found that at twelve and twenty-four weeks after IGF-1 treatment, the proportion of patients showing hearing improvement was 48 percent and 56 percent, respectively. No side effects were observed. The average recovery in pure tone audiometry thresholds over five frequencies tested was 11.9 decibels (43). This was comparable to intratympanic steroid injection (aldosterone) of five to twenty-two decibels.
Figure 2, researchers describe injecting IGF-1 directly into the inner ear by puncturing the eardrum and proceeding to deposit a dose in the cochlea’s interior (39). Over the last decade in Japan, Professor Nakagawa and his large research group have demonstrated that IGF-1 dissolved in hydrogel will absorbed into the cochlea’s hair cells, preventing their apoptosis. IGF-1, the ubiquitous repair hormone, may even rebuild damaged hair cells in the cochlea, subsequently reversing hearing loss partially.

Figure 3: Professor Takayuki Nakagawa of Kyoto Universityhas written 203 publications concerning hearing loss and hormones.
In a 2014 clinical study of 120 patients (43), Nakagawa’s group found that 66.7% of IGF-1-treated patients showed improved hearing versus 53.5% of dexamethasone-treated patients. It was assumed that IGF-1 intratympanic treatment actually repaired hair cells even though this assumption conflicts with the traditional belief that hair cells are postmitotic. In other words, once hair cells die from apoptosis, they should not regenerate (43). However, Professor Nakagawa found this to be incorrect. Hair cells are protected and can regenerate with IGF-1 as indicated in Figure 4.
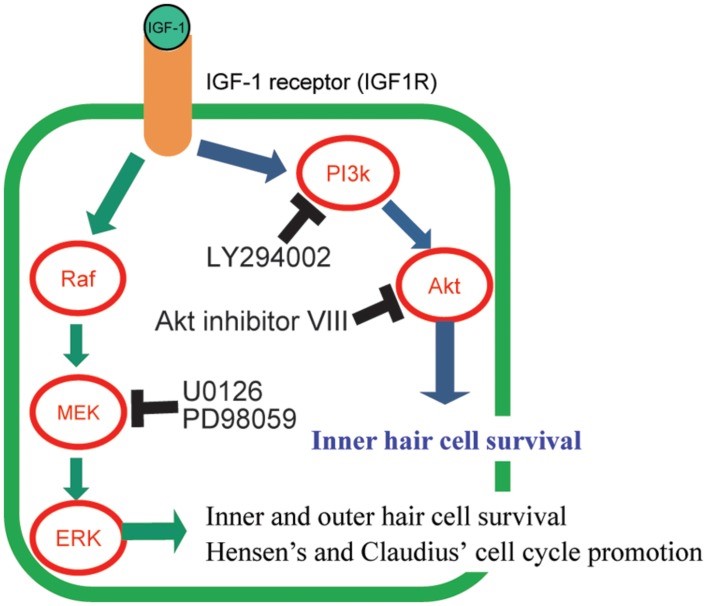
Figure 4:Graphical summary of the signaling pathways of IGF-1 and its downstream effectors and its effect on the protection of hair cells. Hair cells (HCs) maintained by IGF-1 were changed using several specific inhibitors (LY294002, Akt inhibitor VIII, U0126, and PD98059) of the downstream molecules in IGF-1 signaling pathways. IGF-1 protects HCs against aminoglycosides through activation of both the MEK/ERK and PI3K/AKT pathways. AKT is involved in the survival of only inner HCs. MEK/ERK causes cell cycle promotion in Hensen’s and Claudius’s cells (41).
In his natal mouse study from 2013, Nakagawa’s group found that “treatment with IGF-1 maintains hair cell numbers in mammalian cochlea after various types of hair cell injuries by activating two major pathways downstream of IGF-1 signaling” (42).
Lastly, Nakagawa and his researchers demonstrated that even in totally deaf patients, hearing improves by at least ten decibels with IGF-1/hydrogel treatment (41).
Significant Advance in Word Recognition
I have extended Professor Nakagawa’s experiments. Figure 5 shows two audiograms, both the left and right ear, taken in January (black) and May (orange) of 2015. During the intervening five months, a patient applied growth hormone and IGF-1 dissolved in hydrogel in both ears at bedtime. The orange marks show that hearing improved by as much as thirty decibels, but it did not return to normal. Not shown is the fact that word recognition also improved significantly. Dr. Nakagawa and his researchers did not test for this effect. It was also significant that near-normal hearing was achieved when this mixture was applied to the ear canals on alternate days rather than every day. As in the case of many hormones, they must be allowed to cycle between high and low concentrations in the body in order to achieve maximum effect. Also, note that IGF-1 was combined with human growth hormone according to Dr. Thierry Hertoghe’s recommendation (7).
I conclude that growth hormone combined with IGF-1 in hydrogel causes improved word recognition, whereas IGF-1 alone only causes improved sound volume. Thus, if a patient only desires increased sound volume, I recommend treating with only aldosterone and/or IGF-1.
If one treats the ear canals topically at bedtime, one may awaken the following morning with somewhat elevated noise from tinnitus. This is an indication that this mixture has worked to improve both sound volume and word recognition, making hearing aids temporarily unnecessary.
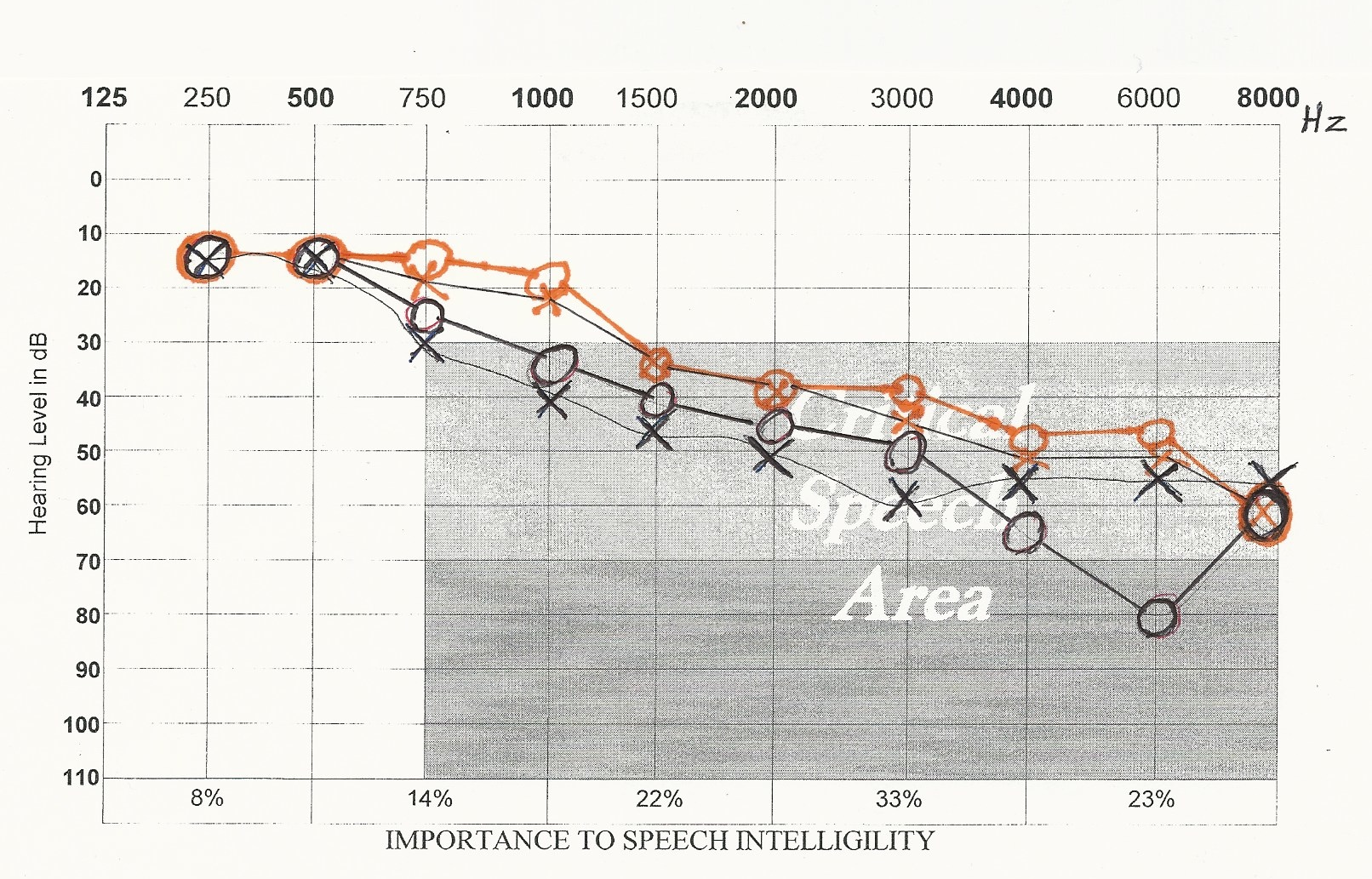
Figure 5: The black audio-graph recordings were taken in January 2015 of both the untreated left and right ear. The orange audiograph recordings were taken in May 2015 after four months of treatment with IGF-1 and growth hormone dissolved in hydrogel.
Conclusion
Aldosterone, growth hormone, and IGF-1 are promising medications for hearing loss. IGF-1 activates both its downstream signaling pathways and the MEK/ERK and PI3K/AKT pathways in the cochlea. IGF-1 protects hair cells from various injuries to the inner ear caused by noise exposure, ischemia, and drug treatment. A clinical trial of IGF-1 treatment on human hearing loss patients, as well as in vivo animal experiments, has confirmed its efficacy against hair cell injuries.
Addendum Clinical Note: A Quick Test for Hormone Deficiency
In 2016 I devised a test to determine a growth hormone/IGF-1 deficiency in patients. When patients over the age of fifty first visit me and want to know the benefits of hormone corrective therapy, I tell them to read my book, Stay 40. I then demonstrate my therapy by applying topically a small dose of HGH plus IGF-1 dissolved in hydrogel to their ear canals. I take a baseline audiogram before treatment and a second audiogram a few hours later.
I ask the patients whether they notice any changes in their hearing, such as improved sound volume and word recognition, and the answer is usually a resounding yes!
References
- Verne, J., 1996, “Paris in the Twentieth Century,” translated US edition by Random House, NY, NY.
- Preston, B., Jan. 8, 2016, “Insurers Brace for a Self-Driving Future (and a Fading Need for Insurance),”New York Times, p. B3.
- A4M or American Academy of Anti-Aging Medicine’s website at a4m.com., then click on mission statement.
- Lin F. R., Thorpe R., Gordon-Salant S., Ferrucci L., 2011, Hearing loss prevalence and risk factors among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 66, pp. 582–590.
- Marazita M. L., Ploughman L. M., Rawlings B., Remington E., Arnos K. S., Nance W. E. (1993). Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am. J. Med. Genet. 46, pp. 486–49.1
- Ruben R. J., 1967, Development of the inner ear of the mouse: a radio-autographic study of terminal mitoses. Acta Otolaryngol. Suppl. 220, pp. 221–244.
- Hertoghe, T. May, 2010. The Hormone Handbook 2nd edition, International Medical Books/ Archimedial, Luxemburg.
- Wright, J.V. Oct. 2008. Don’t Go Deaf, Blind, or Lose Your Mind! Nutrition & Hearing, Vol. 15, Issue 8, pp. 1-7.
- Tadros, SF et al, Nov. 2005. A Possible Protective Hormone against Presbycusic. Hearing Research, 209 (1-2), pp. 10-18.
- Trune, D.R. and Kempton, J.B, May 2001, Aldosterone and Prednisone Control of Cochlear Function in MRL, MpJ-Fas (1pr) Autoimmune Mouse Ear. Hearing Research, 155 (1-2), pp. 9-20
- Trune, D.R. et al, Feb. 2006. Mineralocorticoid Receptor Mediates Glucocorticoid Treatments Effects In The Autoimmune Mouse Ear. Hearing Research, 212 (1-2), pp.22-32.
- Fredholm, L. March 2008. Inreljud. Forsking och Framsteg, pp. 46-52.
- Langer R., Vacanti J. P., 1993, Tissue engineering. Science 260, pp. 920–926.
- Cohen S., 1962, Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 237, pp. 1555–1562.
- Armelin H. A.,1973, Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc. Natl. Acad. Sci. U.S.A. 70, pp. 2702–2706.
- Lefebvre P. P., Malgrange B., Thiry M., Van De Water T. R., Moonen G., 2000, Epidermal growth factor upregulates production of supernumerary hair cells in neonatal rat organ of corti explants. Acta Otolaryngol. 120, pp. 142–145.
- Laron Z., 1999, Somatomedin-1 (recombinant insulin-like growth factor-1): clinical pharmacology and potential treatment of endocrine and metabolic disorders. BioDrugs 11, pp. 55–70.
- Kato H., Faria T. N., Stannard B., Roberts C. T., Jr., Leroith D. ,1994, Essential role of tyrosine residues 1131, 1135, and 1136 of the insulin-like growth factor-I (IGF-I) receptor in IGF-I action. Mol. Endocrinol. 8, pp. 40–50.
- Pagès G., Lenormand P., L’Allemain G., Chambard J. C., Meloche S., Pouysségur J., 1993, Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. U.S.A. 90, pp. 8319–8323.
- Holmström T. H., Tran S. E., Johnson V. L., Ahn N. G., Chow S. C., Eriksson J. E., 1999, Inhibition of mitogen-activated kinase signaling sensitizes HeLa cells to Fas
- receptor-mediated apoptosis. Mol. Cell. Biol. 19, pp. 5991–6002.
- Laviola L., Natalicchio A., Giorgino F., 2007, The IGF-I signaling pathway. Curr. Pharm. Des. 13, pp. 663–669.
- Werner H., Leroith D. (2014). Insulin and insulin-like growth factor receptors in the brain: physiological and pathological aspects. Eur. Neuropsychopharmacol. [Epub ahead of print] 10/1016.
- Woods K. A., Camacho-Hubner C., Savage M. O., Clark A.J., 1996, Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N. Engl. J. Med. 335, pp. 1363–1367.
- Bonapace G., Concolino D., Formicola S., Strisciuglio P., 2003, A novel mutation in a patient with insulin-like growth factor 1 (IGF1) deficiency. J. Med. Genet. 40, pp. 913–917.
- Walenkamp M. J., Karperien M., Pereira A. M., Hilhorst-Hofstee Y., Van Doorn J., Chen J. W., et al., 2005,
- Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J. Clin. Endocrinol. Metab. 90, pp. 2855–2864.
- Attias J., Zarchi O., Nageris B. I., Laron Z., 2012, Cochlear hearing loss in patients with Laron syndrome. Eur. Arch. Otorhinolaryngol. 269, pp. 461–466.
- Barrenas M., Landin-Wilhelmsen K., Hanson C., 2000, Ear and hearing in relation to genotype and growth in Turner syndrome. Hear. Res. 144, pp. 21–28.
- Johnson K. R., Marden C. C., Ward-Bailey P., Gagnon L. H., Bronson R. T., Donahue L. R., 2007, Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol. Endocrinol. 21, pp. 1593–1602.
- Okano T., Xuan S., Kelley M. W., 2011, Insulin-like growth factor signaling regulates the timing of sensory cell differentiation in the mouse cochlea. J. Neurosci. 31, pp. 18104–18118.
- Kopke R. D., Jackson R. L., Li G., Rasmussen M. D., Hoffer M. E., Frenz D. A., et al., 2001, Growth factor treatment enhances vestibular hair cell renewal and results in improved vestibular function. Proc. Natl. Acad. Sci. U.S.A. 98, pp. 5886–5891.
- Lee K. Y., Nakagawa T., Okano T., Hori R., Ono K., Tabata Y., et al., 2007, Novel therapy for hearing loss: delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel. Otol. Neurotol. 28, pp. 976–981.
- Iwai K., Nakagawa T., Endo T., Matsuoka Y., Kita T., Kim T. S., et al., 2006, Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope 116, pp. 529–533. 34. Fujiwara T., Hato N., Nakagawa T., Tabata Y., Yoshida T., Komobuchi H., et al., 2008, Insulin-like growth factor 1 treatment via hydrogels rescues cochlear hair cells from ischemic injury. Neuroreport 19, pp. 1585–1588.
- Hakuba N., Gyo K., Yanagihara N., Mitani A., Kataoka K., 1997, Efflux of glutamate into the perilymph of the cochlea following transient ischemia in the gerbil. Neurosci. Lett.230, pp. 69–71.
- Koga K., Hakuba N., Watanabe F., Shudou M., Nakagawa T., Gyo K., 2003, Transient cochlear ischemia causes delayed cell death in the organ of Corti: an experimental study in gerbils. J. Comp. Neurol. 456, pp. 105–111.
- Hayashi Y., Yamamoto N., Nakagawa T., Ito J., 2013, Insulin-like growth factor 1 inhibits hair cell apoptosis and promotes the cell cycle of supporting cells by activating different downstream cascades after pharmacological hair cell injury in neonatal mice. Mol. Cell. Neurosci. 56, pp. 29–38.
- Nakagawa T., Sakamoto T., Hiraumi H., Kikkawa Y. S., Yamamoto N., Hamaguchi K., et al. (2010). Topical insulin-like growth factor 1 treatment using gelatin hydrogels for glucocorticoid-resistant sudden sensorineural hearing loss: a prospective clinical trial. BMC Med. 8:76 10.1186/1741-7015-8-76.
- Nakagawa T., Ogino-Nishimura E., Hiraumi H., Sakamoto T., Yamamoto N., Ito J. (2012). Audiometric outcomes of topical IGF1 treatment for sudden deafness refractory to systemic steroids. Otol. Neurotol. 33, pp. 941–946.
- Nakagawa, T. et al, Oct. 2007, Novel therapy for hearing loss: delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel.Otol. Neurotol. 28(7), pp. 976-981.
- Nakagawa, T. et al, Nov. 2014, A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 19(12). Pp. 219-224.
- Hayashi Y, Yamamoto N, Nakagawa T, Ito J, Sept 2013, Insulin-like growth factor 1 inhibits hair cell apoptosis and promotes the cell cycle of supporting cells by activating different downstream cascades after pharmacological hair cell injury in neonatal mice, Mol Cell Neurosci, 56, pp. 29-38.
- Yamamoto N, Nakagawa T, Ito J, Sept 2014, Application of insulin-like growth factor-1 in the treatment of inner ear disorders, Front Pharmacol, 10(5), pp. 208-2113.

